how many electrons in the f orbital|2.4 Electron Configurations : Tagatay Learn about the shapes, sizes and capacities of s,p,d,f orbitals, the regions of space where electrons are most likely to be found. Find out how many electrons can s,p,d,f orbitals hold and see examples of electron .
Highlighted features. Scalability via a server multi-client architecture: multiple clients in the same or in different nodes can control different actors.; Flexible API: CARLA exposes a powerful API that allows users to control all aspects related to the simulation, including traffic generation, pedestrian behaviors, weathers, sensors, and much more. .
PH0 · s,p,d,f Orbitals
PH1 · The periodic table, electron shells, and orbitals
PH2 · How many electrons can an orbital of type f hold?
PH3 · How many electrons can an f orbital have?
PH4 · For s, p, d, and f orbitals, how many electrons can each hold?
PH5 · Electronic Orbitals
PH6 · Electron Configuration for Fluorine (F, and F– ion)
PH7 · Atomic orbital
PH8 · 8.3: Electron Configurations
PH9 · 2.4 Electron Configurations
Review – Emirates a380 First Class: Is it worth $10,000? Free Pick up/Drop off Service from the Airport. In Business Class and First Class, Emirates includes a free pick up/drop off service from the Airport. We were not eligible for this service? Why? Because we booked our Emirates a380 tickets through Qantas using a code share.
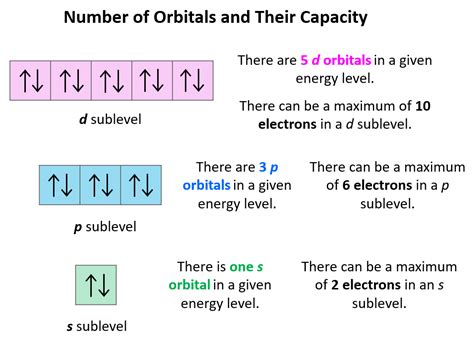
how many electrons in the f orbital*******This means that the s orbital can contain up to two electrons, the p orbital can contain up to six electrons, the d orbital can contain up to 10 electrons, and the f orbital can contain up to 14 electrons.Magnetic Spin, Magnetism, and Magnetic Field Lines. An atom with unpaired .
As shown by the graphs, electrons of the s orbital are found closer to the nucleus .

Introduction. Before assigning the electrons of an atom into orbitals, one must . Learn how many electrons each subshell can hold, from s with 2 to f with 14. See the answer, explanation, and link to the source on Socratic.Learn about the shapes, sizes and capacities of s,p,d,f orbitals, the regions of space where electrons are most likely to be found. Find out how many electrons can s,p,d,f orbitals hold and see examples of electron .
Oxygen (atomic number 8) has a pair of electrons in any one of the 2 p orbitals (the electrons have opposite spins) and a single electron in each of the other two. Fluorine (atomic number 9) has only .
How many electrons can an orbital of type f hold? A. 6. B. 10. C. 2. D. 14. E. 1. Since there can be [-ℓ, ℓ] orientations and since the orbital type f has ℓ = 3, we should have 7 .Each orbital in an atom is characterized by a set of values of the three quantum numbers n, ℓ, and ml, which respectively correspond to the electron's energy, its orbital angular momentum, and its orbital . Answer link. The f orbital has 7 sub levels with the possibility of two electrons in each suborbital. Therefore, the f orbital can hold 14 electrons.
Learn how atoms organize their electrons in shells and orbitals, and how this affects their chemical reactivity. The web page explains the Bohr model, the periodic table, and the .1 Answer. BRIAN M. May 26, 2014. The f orbital has 7 sub levels with the possibility of two electrons in each suborbital. Therefore, the f orbital can hold 14 electrons.Figure 9.6.5 9.6. 5: Electrons are added to atomic orbitals in order from low energy (bottom of the graph) to high (top of the graph) according to the Aufbau principle. Principle energy levels are color coded, while sublevels are grouped together and each circle represents an orbital capable of holding two electrons.Each orbital can hold two electrons. They are also known as atomic orbitals. Atomic orbitals come in different shapes, depending on how much energy and angular momentum is associated with that orbital. . The letter tells you which orbital it is, eg s, p, d or f The superscript number tells you how many electrons are in that orbital 1s^2 means 2 electrons are in the 1s orbital 1s^2 2s^2 .
F orbitals are the orbitals that, in total, have the affinity to accommodate 14 electrons in them. The shape of the f orbital is tetrahedral. Though the shape of the f orbital is more complex than the other orbitals, the rule of filling the orbital remains the same as that of p and the d orbitals. The alignment of the electrons is also found to . How many electrons can s,p,d,f hold? Chemistry Electron Configuration s,p,d,f Orbitals. 1 Answer Junaid Mirza May 9, 2018 #2, 6, 10, 14# respectively. Explanation: If . How many electrons can an f orbital have? How many electrons can there be in a p orbital? .
how many electrons in the f orbital 2.4 Electron Configurations These are arbitrarily given the symbols px, py and pz. This is simply for convenience; the x, y, and z directions change constantly as the atom tumbles in space. Figure 3: Hydrogen's electron - the 2p orbitals. The p orbitals at the second energy level are called 2p x, 2p y and 2p z. There are similar orbitals at subsequent levels: 3p x, 3p y .The four chemically important types of atomic orbital correspond to values of l = 0, 1, 2, and 3. Orbitals with l = 0 are s orbitals and are spherically symmetrical, with the greatest probability of finding the electron occurring at the nucleus. All orbitals with values of n > 1 and l = 0 contain one or more nodes.Notice that the 1s orbital has the highest probability. This is why the hydrogen atom has an electron configuration of 1s 1. 2) Orbitals are combined when bonds form between atoms in a molecule. There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental).7f atomic orbitals. For any atom, there are seven 7f orbitals. The f-orbitals are unusual in that there are two sets of orbitals in common use.The first set is known as the general set, this page.The second set is the cubic set, this page and these might be appropriate to use if the atom is in a cubic environment, for instance. Three of the orbitals are common to .The s sublevel has only one orbital, so max. 2 electrons can be present. The p sublevel has 3 orbitals, so max. 6 electrons can be present. The d sublevel has 5 orbitals, so max. 10 electrons can be present. And the .
Fourteen would be the maximum number of electrons across an entire f-type sub-shell, but the question only asks about one orbital. answered. 11.9k. The question specifically ask that no.of electron an orbital of f subshell can hold.. As we know that f subshell contain 7 orbital and each orbital can hold maximum 2 electons so correct answer . Quantum numbers for the first four shells. Calculates number of orbitals and number of electrons in different kinds of orbitals for n = 1 to 4. Explains that only two electrons are .
T he number of electrons in each orbital is explained in the following ways:. Each subshell contains a distinct number of orbitals according to the Principal Quantum number(n).Each orbital can occupies two electrons in opposite spins according to .2.4 Electron Configurations The four chemically important types of atomic orbital correspond to values of ℓ = 0, 1, 2, and 3. Orbitals with ℓ = 0 are s orbitals and are spherically symmetrical, with the greatest probability of finding the electron occurring at the nucleus. All orbitals with values of n > 1 and ell = 0 contain one or more nodes. On the far left of Figure 3.6.1 3.6. 1 are the highest energy electromagnetic waves. These are called gamma rays and can be quite dangerous, in large numbers, to living systems. The next lower energy form of electromagnetic waves are called x-rays. Most of you are familiar with the penetration abilities of these waves.
how many electrons in the f orbital The Azimuthal Quantum Number. The second quantum number is often called the azimuthal quantum number (l).The value of l describes the shape of the region of space occupied by the electron. The allowed values of l depend on the value of n and can range from 0 to n − 1: \[l = 0, 1, 2,., n − 1 \label{6.5.2}\]
If an electron has an orbital angular momentum of 7.892 x 10-34 Js, what is the orbital quantum number for the state of the electron? How many valence electrons does promethium have? How many orbitals are contained in the third principal level (n=3) of .
Easily convert Tether to Philippine Peso with our cryptocurrency converter. 1 USDT is currently worth ₱56.48. . Tether (USDT) is an Ethereum token that is pegged to the value of a U.S. dollar (also known as a stablecoin). Tether’s issuer claims that USDT is backed by bank reserves and loans which match or exceed the value of USDT in .
how many electrons in the f orbital|2.4 Electron Configurations